January 12, 2023
Author: Manish Verma
Editor: Dr. Jitendra Kumar Sinha
Hello there, fellow brain geeks. We know how much you enjoy learning more and more interesting stuff about the brain. But the brain isn’t the only thing which hides a plethora of mysteries. Even the blood in our bodies, which travels from cell to cell and communicates, can teach us a lot about what’s going on in our bodies. As if it were a doorway into our health. Researchers have been trying harder than ever to find biomarkers that might help us detect the development or presence of certain ailments. These biomarkers will be critical in the development of new tailored therapeutics. Blood biomarkers are non-invasive, easy to obtain, and simple to employ in normal therapy, making them a promising contender in the search for medications with effective therapeutic techniques. A group of researchers recently moved on to explore for signs of Alzheimer’s disease in blood. Let’s have a peek at what they discovered.
Alzheimer’s disease is a chronic and progressive neurodegenerative brain disorder that affects approximately 30 million individuals worldwide. Given the variety of variables contributing to AD development, there is currently no effective therapy.
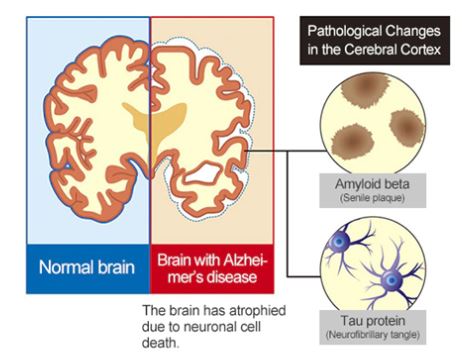
Currently, physicians employ the criteria established in 2011 by the National Institute on Aging and the Alzheimer’s Association to diagnose Alzheimer’s disease. The AT(N) (amyloid, tau, neurodegeneration) research paradigm offers a unified framework that highlights pathophysiological evidence of amyloid beta (A), tau (T), and neurodegeneration (N) for the classification and staging of Alzheimer’s disease. However, the approach presently relies on well-established CSF and neuroimaging biomarkers, which face significant obstacles from an economic, practical, and logistical standpoint that restrict their broad use. Particularly in situations that call for low-cost, high-throughput investigations for biological evidence of Alzheimer’s disease. For instance, biomarker screening at the primary care level might be advantageous due to the shortage of dementia experts in many hospital systems to expedite patient treatment, including referrals to expert clinicians.
In the amyloid/tau/neurodegeneration [A/T/ (N)] paradigm for Alzheimer’s disease, blood-based biomarkers for amyloid beta and phosphorylated tau have strong diagnostic accuracies and agreement with their corresponding CSF and neuroimaging biomarkers. Meeting two of the three requisite checkmarks to confidently diagnose Alzheimer’s. However, neurofilament light, a blood-based neurodegenerative marker, is not specific to Alzheimer’s disease, and total-tau has very little correlation with CSF total-tau. According to recent research, blood total-tau arises mostly from peripheral, non-brain sources.
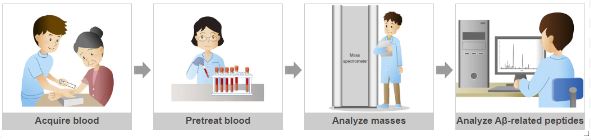
Using a blood sample, a team of neuroscientists lead by a researcher from the University of Pittsburgh School of Medicine devised a test to identify a novel marker of Alzheimer’s disease neurodegeneration.
Karikari and colleagues aimed to address this problem by developing an anti-tau antibody that binds brain-derived tau while avoiding the peripherally produced ‘large tau’ variant. This antibody was utilized to construct an ultrasensitive blood-based test for brain-derived tau, that was validated in five distinct cohorts (n=609), including a blood-to-autopsy, CSF biomarker-classified, and memory clinic cohorts.
The experiments revealed that levels of BD-tau measured in blood samples from Alzheimer’s patients using the novel assay corresponded with levels of tau in the CSF and effectively distinguished Alzheimer’s from other neurodegenerative disorders. According to brain autopsy studies, the researchers discovered a correlation between BD-tau levels and also how extensive amyloid plaques and tau tangles were present in the brain tissue.
The foregoing results provide a glimmer of optimism. However, there is a need to validate the test in a wider spectrum of individuals, including those with diverse racial and ethnic origins, varying degrees of memory loss, or other probable dementia symptoms.
In the future, the researchers hope to carry out extensive clinical validation of blood BD-tau in a variety of study groups, including those that draw participants from the community, memory clinics, and people of various racial and cultural origins. Furthermore, these trials will involve both older persons with no biochemical indications of Alzheimer’s disease and those in various stages of the illness. These initiatives will pave the road for the commercial release of BD-tau for wider clinical and prognostic usage and are essential for ensuring that the biomarker results are generalizable to individuals from all backgrounds.
Abbreviation:
AD – Alzheimer’s Disease
NFL – Neurofilament
BD-tau – Brain Derived-tau
CSF – Cerebrospinal Fluid
AT(N) – Amyloid-beta Tau (Neurodegeneration)
REFERENCES:
- Gonzalez-Ortiz, F., Turton, M., Kac, P. R., Smirnov, D., Premi, E., Ghidoni, R., Benussi, L., Cantoni, V., Saraceno, C., Rivolta, J., Ashton, N. J., Borroni, B., Galasko, D., Harrison, P., Zetterberg, H., Blennow, K., & Karikari, T. K. (2022). Brain-derived tau: a novel blood-based biomarker for Alzheimer’s disease-type neurodegeneration. Brain : a journal of neurology, awac407. Advance online publication. https://doi.org/10.1093/brain/awac407
- Delaby, C., Hirtz, C., & Lehmann, S. (2022). Overview of the blood biomarkers in Alzheimer’s disease: Promises and challenges. Revue neurologique, S0035-3787(22)00792-5. Advance online publication. https://doi.org/10.1016/j.neurol.2022.09.003
- Ghosh, S., Durgvanshi, S., Agarwal, S., Raghunath, M., & Sinha, J. K. (2020). Current Status of Drug Targets and Emerging Therapeutic Strategies in the Management of Alzheimer’s Disease. Current neuropharmacology, 18(9), 883–903. https://doi.org/10.2174/1570159X18666200429011823