September 5, 2023
Author: Harshita Sharma
Editor: Dr. Jitendra Kumar Sinha
Imagine a world where nights are endless, and sleep remains an elusive dream. Insomnia, a sleep disorder that plagues countless individuals, delves deeper into the intricate realm of neuropharmacology. Beyond restless nights and weary days, lies a captivating story of how our brain’s intricate chemistry orchestrates our sleep-wake cycle. Step into a world where biogenic amines dance, serotonin unveils its secrets, and dopamine takes center stage in the saga of insomnia. Join us as we embark on a GloNeuro journey through the enigmatic pathways of our brain, deciphering the intricate codes that govern our quest for rest. Welcome to the realm of Neuropharmacology of insomnia – where every neuron has a tale to tell, and every neurotransmitter unveils a mystery waiting to be solved.
Insomnia is classified as a sleep disorder characterized by persistent difficulty in falling asleep or staying asleep for an extended period. This condition is often associated with significant impairment in the overall quality of life.
During Insomnia, individuals often experience heightened arousal throughout the entire day. This arousal observed in insomnia is characterized by two models: Cognitive and Physiological. Evidence of this heightened arousal is manifested through the intense activation of the stress response system. Insomnia disrupts both physical and cognitive functioning, leading to a variety of impaired daytime activities. Insomnia can be classified into two categories: short term, which can be triggered by stress or environmental changes, and chronic insomnia defined as occurring three or more nights per week.
Neuropharmacology:
Neuropharmacology of insomnia encompasses different components that impact insomnia in distinct ways. These components are mentioned below:
- Biogenic amines:
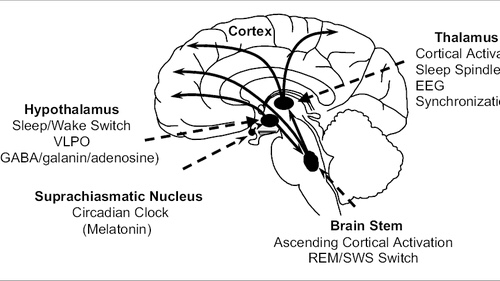
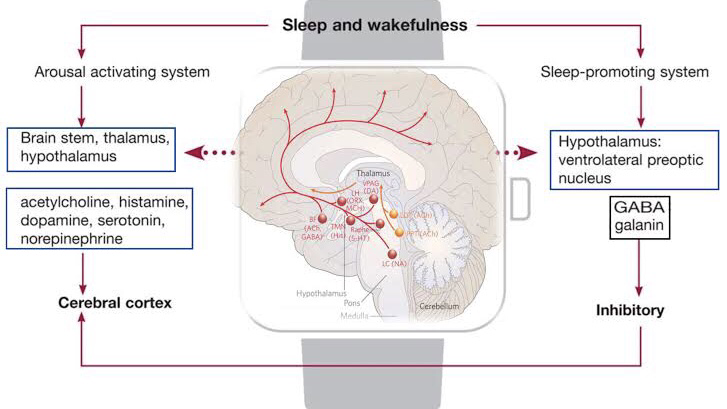
Different monoamines play a role in heightening arousal. Neurons producing serotonin in the dorsal raphe nucleus, norepinephrine in the locus coeruleus, and histamine in the tuberomammillary nucleus exhibit rapid discharges during arousal.
- Serotonin:
Studies on rats have demonstrated that serotonin enhances wakefulness. Specifically, serotonin acting on certain receptors plays a pivotal role in suppressing REM sleep. Peaks in serotonin release occur in the preoptic area and dorsal raphe nucleus during arousal. Electrical stimulation of the dorsal raphe nucleus increases arousal. Administering agonists targeting specific serotonin receptors leads to heightened arousal and decreased sleep.
- Norepinephrine:
Noradrenergic cells within the locus coeruleus inhibit REM sleep, resulting in increased wakefulness. These cells project to brain regions that regulate arousal, including the Forebrain, thalamus, basal ganglia, hypothalamus, and cortex. Administering noradrenaline or its alpha and beta receptor agonists to the medial preoptic or septal area enhances wakefulness. However, blocking alpha- and beta-adrenergic receptors, commonly used to treat hypertension, can lead to sleep disturbances.
- Histamine:
Histamine-containing cell bodies within the tuberomammillary nucleus of the posterior hypothalamus send out projections throughout the brain. Lesion studies of the posterior hypothalamus and single unit recordings indicate that the tuberomammillary nucleus promotes arousal. Lowering histamine levels by inhibiting synthesis significantly decreases wakefulness and increases NREM sleep in rats and cats, respectively. Research on specific H1 receptors suggests that histamine signaling through these receptors contributes to wakefulness.
- Dopamine:
Stimulants like cocaine, methylphenidate, and amphetamine enhance arousal and counter hypersomnia by increasing endogenous dopamine levels. In the human brain, in vivo imaging studies indicate that sleep deprivation raises dopamine levels. Dopaminergic neurons regulating wakefulness are situated in the Ventral tegmental area and substantia nigra pars compacta. Some dopaminergic neurons also exist in the ventrolateral periaqueductal gray, functioning during arousal. Dopamine enhances wakefulness; knockout mice lacking the dopamine transporter show increased wakefulness and reduced NREM sleep compared to controls. Intracerebroventricular delivery of D2 or D1 receptor agonists enhances wakefulness. Systemic administration of D-amphetamine in rats boosts arousal and consequently decreases both REM and NREM sleep.
- Glutamate:
As a primary excitatory neurotransmitter in the human brain, glutamate’s role in arousal and sleep regulation is not fully understood. Studies indicate increased glutamate concentrations in specific rat cortex regions during REM sleep and arousal, accompanied by decreased NREM sleep.
- Hypocretin-1 and 2:
Considered crucial for sustaining arousal, Hypocretin-1&2 neurons reside in the dorsolateral hypothalamus, projecting to brain areas modulating wakefulness. In the cat’s hypothalamus, Hypocretin-1 levels are higher during arousal and REM sleep than NREM sleep. Administration of Hypocretin-1 to various brain regions enhancing wakefulness, solidifying its role as an arousal promoting neuropeptide.
- Leptin and Ghrelin:
Leptin and Ghrelin’s role in sleep has been studied extensively. Lower Leptin and higher Ghrelin levels are linked to shorter sleep duration in humans. Locally administering Ghrelin to different brain regions in rats increases wakefulness.
- Opioids:
Opioids, a class of drugs with sleep-disrupting side effects, affect sleep stages. Morphine decreases stage III & IV NREM sleep while increasing stage II NREM sleep. Administration of morphine into the pontine reticular formation of rats or cats enhances wakefulness and decreases REM sleep.
- GABA:
GABA, the primary inhibitory neurotransmitter in the human brain, plays a role in arousal and sleep. In the pontine reticular formation, GABA levels are higher during arousal compared to REM sleep. Reducing extra cellular GABA levels in the rat’s pontine reticular formation decreases arousal and increases sleep.
.
- Acetylcholine:
The first identified neurotransmitter, acetylcholine, is integral to producing REM sleep and activating brain states associated with arousal. Neurons in the LDT/PPT transmit to various arousal-promoting brain regions. Cortical acetylcholine secretion is higher during REM sleep and arousal than during NREM sleep. Enhanced cortical activation during REM sleep and arousal is attributed to basal forebrain cholinergic transmission.
Reference:
- Roth T. (2007). Insomnia: definition, prevalence, etiology, and consequences. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine, 3(5 Suppl), S7–S10.
- Watson, C. J., Baghdoyan, H. A., & Lydic, R. (2010). Neuropharmacology of Sleep and Wakefulness. Sleep medicine clinics, 5(4), 513–528. https://doi.org/10.1016/j.jsmc.2010.08.003
- Hamblin J. E. (2007). Insomnia: an ignored health problem. Primary care, 34(3), 659–viii. https://doi.org/10.1016/j.pop.2007.05.009
Explore New Articles from GloNeuro