August 12, 2023
Author: Anisha Bhola
Editor: Dr. Jitendra Kumar Sinha
Name of Technique: Hematoxylin and Eosin staining.
Used along with:
Immunohistochemistry (IHC), Immunofluorescence, Electron Microscopy, Frozen Section Analysis, and In situ Hybridization (ISH).
Purpose:
Tissue Identification, Visualisation of Cellular Morphology, Diagnosis of Diseases, Tumor Grading, and
Research and Education.
Studying the constituent cells and the relationship of the tissue along with the morphology can be done once the tissue section is stained. Staining solutions can be prepared with the help of different dyes (Synthetic or Natural). H&E is widely used by pathologists as it helps to visualize cellular and structural details.
H&E demonstrate a broad range of extracellular matrix, cytoplasmic and nuclear feature. Cytopathology and histopathology laboratories use the stain to highlight the biological tissue’s structure and then visualize it with the aid of a microscope. To examine the organelles, cell population, or bulk tissues staining techniques are used.
The study of brain tissues relies heavily on the staining techniques used for examination and analysis. Among these techniques, Hematoxylin and Eosin staining (H&E staining) holds a prominent position as one of the most widely utilized and indispensable methods. H&E staining is a histological staining technique enabling researchers to visualize and distinguish different components of brain tissue.
Embedded tissues which are paraffin-fixed, frozen sections along with fine needle aspirates are a few of the many areas of any histopathology laboratory in which Hematoxylin and eosin stains are used. it is very crucial to first understand the components of the stains before understanding what makes a well-stained slide.
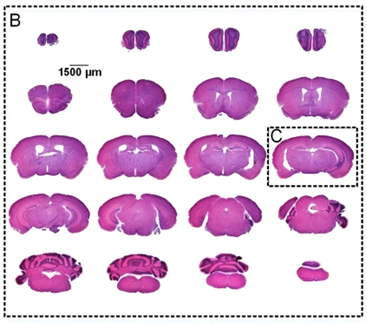
[Sourced fromLi Y., 2018]
The nuclear details of any cell are illustrated with Hematoxylin. The time duration the sample needs is Hematoxylin is quite important along with the amount of DNA in the nuclei for the depth of coloration.Hematoxylin, a basic dye, imparts a blue-purple color to acidic components, such as cell nuclei. Hematoxylum campechianum is the logwood tree from which heartwood is obtained which produces Hematoxylin. Hematein is formed when Hematoxylin undergoes oxidation and specific metal ions like Al (Aluminium) and Fe (Iron) make a complex with the compound formed by Hematein. The staining of cell nuclei before examining them under a microscope is done with this Metal-Hematein complex.
Eosin, an acidic dye, gives a pink or red color to basic components, like cytoplasm and extracellular matrix. The distinct colors resulting from these dyes facilitate precise microscopic examination and analysis of brain tissue architecture. Hematoxylin is coupled with a mordant before staining which improves the ability of the hematein to attach to the anionic (negatively charged) as the hematin’s positive charge is strengthened with mordants. Aluminum ammonium sulfate (alum) is the most common mordant used in the routine histology lab. Mordant-associated Hematoxylin is typically known as special stains as more detailed tissue demonstration can be done using them. Weigert’s is one of the best mordant-associated hematoxylin stains.
There are 3 types of alum hematoxylin:
- Mayer’s hematoxylin is used as a nuclear counterstain for special stains and immunohistochemistry.
- Harris hematoxylin is used for staining cytology specimens and histology. The staining tends to provide clear nuclear detail.
- Gill’s hematoxylin is available in different concentrations and the nature of Gills is such that extra-nuclear staining may occur.
Gained much knowledge about the nuclear stain. Now let us explore cytoplasmic component staining.
The counterstain, Eosin stains the cytoplasm and tells the difference between the cytoplasm and the nuclei of the cells. Eosin proves to be the best option when it comes to routine staining, as it pairs well with Hematoxylin. Being part of the Xanthene group, Eosin is a naturally anionic dye, making it crucial for staining cytoplasm and extracellular structures. There exist various Eosin stains, including Pink, Red, Orange, and Yellow, among which Eosin Yellow (Y) stands out as the stain most frequently employed in histopathology laboratories and can be used in both alcohol and water. EA65 and EA50 are other Eosin Staining mixtures that are sometimes used primarily for cytology along with Eosin Y which gives brismarck brown, light green, and yellowish staining. A variation of colors from pale blue to pink cytoplasm is obtained by the addition of these two dyes. Eosin staining becomes sharper when it is paired with a small amount of acetic acid. The red which is seen with H&E staining will be increased when Eosin is paired with Phloxine. Hence, Pathologists can incorporate customization, as a large variety of Hematoxylin and Eosin staining is available to get the desired results.
This neuro blog aims to illuminate the importance, procedure, and applications of H&E staining in neurobiology research.
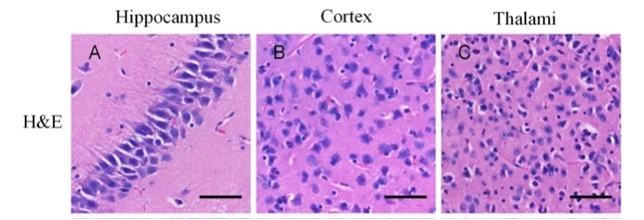
The Brilliant Pros of Hematoxylin and Eosin Staining:
- In research and medical laboratories Hematoxylin and eosin (H&E) staining is widely available and the most commonly used techniques which is performed routinely (Widespread Use).
- The cellular structure within tissues are visualized with great efficiency with the help of H&E staining. Hematoxylin stains nuclei (blue/purple), and eosin stains cytoplasm (pink), providing valuable information about cell morphology and tissue architecture (Visualization of Cellular Structure).
- Staining plays a crucial role in distinguishing different tissue types, including epithelial, connective, muscle, and nervous tissues, thereby assisting pathologists in recognizing both normal and abnormal tissue components (Tissue Classification).
- H&E staining plays a vital role in the diagnosis of different diseases like cancers and inflammatory conditions, enabling pathologists to evaluate disease severity and make predictions about patient outcomes (Diagnosis and Prognosis).
- H&E staining is relatively cost-effective/cheaper, making it accessible to a wide range of medical facilities as compared to other advanced staining and imaging techniques (Cost-Effective).
Beyond the Beauty: Navigating the Limitations of H&E Staining:
- H&E staining doesn’t provide details regarding the genetic alterations as well as the molecular alterations which are needed for proper treatment and diagnosis. It only provides tissue morphology information Limited information).
- Interpreting H&E-stained slides depends on the pathologist’s expertise which can add subjectivity and the possibility of inter-observer differences (Subjectivity).
- When pathologists have to prepare and stain a large number of samples then H&E can be laborious (Time Consuming).
- As years pass by the quality of the information we get from H&E slides may degradeas slides may degrade or fade away (Sample Deterioration).
- H&E staining limits the ability for quantification of molecular and the cellular characteristics. Hence doesnt provide quantitative data (Lack of Quantitative Data).
Factors that can influence the H&E staining process:
- Proper fixation of the tissue is crucial for preserving cellular structures. Over-fixation or under-fixation can affect the staining results (Quality of Tissue Fixation).
- The manner in which the tissue is processed before staining can impact the quality of the final stain. Factors like dehydration, clearing, and embedding need to be carefully executed (Tissue Processing).
- The duration of exposure to hematoxylin and eosin can affect the intensity of staining. Understaining may result in faint details, while overstaining can lead to artifacts (Staining Duration).
- Temperature variations during the staining process can affect the staining outcome. Maintaining consistent temperature levels is essential (Staining Temperature).
- The pH of hematoxylin and eosin solutions influences their chemical reactions with tissue components. Improper pH levels can cause suboptimal staining (pH of Stains).
- The quality and freshness of staining solutions are essential for obtaining consistent and reliable results (Quality of Staining Solutions).
- Proper rinsing of excess stain and controlled drying are necessary to avoid artifacts and uneven staining (Rinsing and Drying Steps).
- The thickness of tissue sections can impact the penetration and uniformity of staining (Section Thickness).
- The quality and calibration of the microscope used to view the stained sections can affect the interpretation of results (Quality of Microscope).
- The expertise of the individual performing the staining procedure plays a significant role in obtaining optimal results (Technician Skill and Experience).
- Contamination of reagents, equipment, or samples can lead to unintended staining artifacts (Contamination).
- Variability in tissue composition and staining characteristics may arise due to intrinsic differences within the tissue (Tissue Heterogeneity).
Preparation of Hematoxylin stain (Harris Hematoxylin)
Reagents:
Hematoxylin:1 gm
95% (v/v) Ethanol:10 ml
Ammonium or Potassium Alum:20 gm
Mercuric Oxide:0.5 gm
Distilled Water:200m
- Start by dissolving 1.0 gm of Hematoxylin in 10 ml of 95% Ethanol using a mortar and pestle.
- Simultaneously, mix 20 gm of Ammonium or Potassium Alum with 200 ml of warm distilled water.
- Once both solutions are warm, combine them and bring the mixture to a boil while stirring constantly.
- Next, add 0.5 gm of Mercuric oxide, and immediately remove the mixture from the heat source.
- Cool it as quickly as possible using running tap water.
- Filter the resulting content and store it in a brown or Amber colored bottle.
The solution will remain stable at room temperature (20°C – 30°C) for several months.
Preparation of Eosin stain (Eosin Y):
Reagents:
Eosin Y:1 gm
Distilled water:80 ml
Ethanol 95% (v/v):320 ml
Glacial Acetic Acid:0.4 ml
- Begin by dissolving 1.0 gm of Yellow eosin in approximately 80 ml of distilled water.
- Then, add 320 ml of 95% (v/v) ethanol to the solution.
- Incorporate 0.4 ml of Glacial Acetic acid and thoroughly mix everything together.
- This solution will remain stable at room temperature (20°C – 30°C) for several months.

[Sourced from Leica Biosystems Knowledge Pathway content]
Hematoxylin and Eosin staining protocol:
Hematoxylin and eosin stain (H&E) – University of Rochester Medical Center.
Available at: https://www.urmc.rochester.edu/MediaLibraries/URMCMedia/musculoskeletal-research/core-services/histology/documents/CMSR-H-E.pdf (Accessed: 29 July 2023).
References:
- Hematoxylin and eosin stain (H&E) – university of rochester medical center. Available at: https://www.urmc.rochester.edu/MediaLibraries/URMCMedia/musculoskeletal-research/core-services/histology/documents/CMSR-H-E.pdf (Accessed: 29 July 2023).
- https://paramedicsworld.com/histopathology-practicals/hematoxylin-eosin-staining-protocol-principle-procedure-results/medical-paramedical-studynotes.
- https://www.leicabiosystems.com/en-in/knowledge-pathway/he-staining-overview-a-guide-to-best-practices/
- Li, Y., Li, N., Yu, X. et al. Hematoxylin and eosin staining of intact tissues via delipidation and ultrasound. Sci Rep 8, 12259 (2018). https://doi.org/10.1038/s41598-018-30755-5